Articles from GSK plc

GSK plc (LSE/NYSE: GSK) today announced new effectiveness data for AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) at RSVVW’26, the 9th Conference of the Respiratory Syncytial Virus Foundation (ReSViNET) in Rome, Italy. GSK is presenting 19 abstracts at the congress and supporting a further 3, reflecting GSK’s leadership in research and prevention of RSV. AREXVY is indicated for the prevention of lower respiratory tract disease (LRTD) caused by RSV in individuals 60 years of age and older, as well as individuals 50 through 59 years of age who are at increased risk for LRTD caused by RSV.
By GSK plc · Via Business Wire · February 18, 2026

GSK plc (LSE/NYSE: GSK) today announced the 2025 recipients of its COiMMUNITY Initiative grant program, awarding a total of $2 million across 16 state, regional and national non-profits working to improve immunization rates across the US. Through COiMMUNITY, GSK invests in local efforts to improve vaccine education and uptake. Now in its third year, the Initiative will offer $3M in grants supporting projects that improve vaccine communication and delivery for people of all ages.
By GSK plc · Via Business Wire · January 21, 2026

GSK plc (LSE/NYSE: GSK) has announced the recipients of the Linked by Lupus: Optimal Care Initiative grant, awarding nearly $1 million to support national, state, and local non-profit organizations working to improve the lives of people impacted by lupus, including systemic lupus erythematosus (SLE) and lupus nephritis (LN).
By GSK plc · Via Business Wire · January 20, 2026

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved Exdensur (depemokimab-ulaa) as an add-on maintenance treatment of severe asthma characterised by an eosinophilic phenotype in adult and pediatric patients aged 12 years and older.
By GSK plc · Via Business Wire · December 16, 2025

GSK plc (LSE/NYSE: GSK) today announced the US Food and Drug Administration (FDA) has approved Blenrep (belantamab mafodotin-blmf) in combination with bortezomib and dexamethasone (BVd) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least two prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory (IMID) agent.
By GSK plc · Via Business Wire · October 23, 2025

GSK plc (LSE/NYSE: GSK) today announced a major update to Vaccine Track, a first-of-its-kind, interactive public data tool for tracking adult immunization activity in the US. Users can now explore vaccination trends across 387 different metro areas* to get insights on adult immunization at the local level. Vaccine Track now also includes a full decade of data, providing a longer look at adult vaccination trends over time. Previously, the site focused on state and national data from 2019 onward. New month-by-month and geographic data are added to the site each quarter.
By GSK plc · Via Business Wire · October 1, 2025

GSK plc (LSE/NYSE: GSK) announced its sponsorship of the 2025 National Senior Games as part of its nationwide Sideline RSV campaign, a health education campaign aimed at helping educate older adults and their loved ones about the risks of respiratory syncytial virus, or RSV. The National Senior Games give adults over 50 across the US the opportunity to stay engaged and active in competitive sports like basketball and swimming, providing an opportune setting to put a focus on healthy aging and prevention and to raise awareness about the risks of RSV. To extend this message beyond the Games, GSK will debut a national television special this fall called Redefining Aging with Senior Athletes, which will feature four Senior Games athletes’ RSV-related stories.
By GSK plc · Via Business Wire · July 24, 2025

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved a prefilled syringe presentation of SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted) for the prevention of shingles (herpes zoster). The new prefilled syringe removes the need to reconstitute separate vials prior to administration, simplifying the vaccine administration process for healthcare professionals.
By GSK plc · Via Business Wire · July 17, 2025

GSK plc (LSE/NYSE: GSK) today announced it has started shipping doses of its trivalent seasonal influenza vaccines to US healthcare providers and pharmacies in preparation for the 2025-26 flu season. This immediately follows a licensing and lot-release approval from the US Food and Drug Administration (FDA). Both FLULAVAL and FLUARIX will be available in a 0.5mL, single-dose, pre-filled syringe and are indicated for people six months and older.
By GSK plc · Via Business Wire · July 10, 2025

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved a 200 mg/mL autoinjector of Benlysta (belimumab), a B-lymphocyte stimulator (BlyS)-specific inhibiting monoclonal antibody, for subcutaneous injection in patients five years of age and older with active lupus nephritis (LN) who are receiving standard therapy. With this approval, GSK is expanding choices for belimumab treatment, offering pediatric lupus nephritis patients and caregivers a first-of-its-kind subcutaneous option that can be administered at home. The 200 mg/mL autoinjector was approved for pediatric patients with active systemic lupus erythematosus (SLE) in 2024.
By GSK plc · Via Business Wire · June 24, 2025

GSK plc (LSE/NYSE: GSK) today announced the launch of the Linked by Lupus: Optimal Care Initiative to help support individuals impacted with lupus, particularly systemic lupus erythematosus (SLE) and lupus nephritis (LN).
By GSK plc · Via Business Wire · June 16, 2025

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved Nucala (mepolizumab) as an add-on maintenance treatment for adult patients with inadequately controlled COPD and an eosinophilic phenotype.
By GSK plc · Via Business Wire · May 22, 2025

GSK plc (LSE/NYSE: GSK) today announced its sponsorship of Pretty Hurts, a gripping new Lifetime Original Movie and the latest extension of GSK’s Ask2BSure public health campaign. Premiering on June 28, 2025, at 8 p.m. ET/7 p.m. CT, Pretty Hurts features a storyline that aims to raise awareness among parents about meningococcal disease, known as meningitis, an uncommon but serious illness that teens and young adults aged 16-23 are at an increased risk for.1,2,3 GSK provided financial and content support for the film, which alerts moms that while many teens have received vaccination against meningitis serogroups A, C, W, and Y, many may be missing meningitis B vaccination.4
By GSK plc · Via Business Wire · May 9, 2025

GSK plc (LSE/NYSE: GSK) is pleased that the Advisory Committee on Immunization Practices (ACIP) voted in favor of recommending the use of RSV vaccines including GSK’s AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) in adults aged 50-59 who are at increased risk for severe RSV disease. This includes people with conditions like COPD, asthma, diabetes, heart disease and those in residential care3. This expands on ACIP’s previous vote in June 2024 to recommend RSV vaccines for adults aged 60-74 who are at increased risk and all adults aged 75 and older. AREXVY is indicated for the prevention of lower respiratory tract disease (LRTD) caused by RSV in individuals 60 years of age and older, as well as individuals 50 through 59 years of age who are at increased risk for LRTD caused by RSV.
By GSK plc · Via Business Wire · April 16, 2025

GSK plc (LSE/NYSE: GSK) today announced that the US Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) has voted to recommend use of PENMENVY (Meningococcal Groups A, B, C, W, and Y Vaccine) as part of the adolescent meningococcal vaccination schedule. Recommendations made by the ACIP are reviewed and, if adopted, are published as official CDC recommendations.
By GSK plc · Via Business Wire · April 16, 2025

GSK plc (LSE/NYSE: GSK) today announced the recipients of its COiMMUNITY Initiative grant program, awarding nearly $2 million to 15 regional, state and national non-profits and community-based groups working to boost adult immunization through improved vaccine education, outreach and access.
By GSK plc · Via Business Wire · April 9, 2025

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved Blujepa (gepotidacin) for the treatment of female adults (≥40 kg) and pediatric patients (≥12 years, ≥40 kg) with uncomplicated urinary tract infections (uUTIs) caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii complex, Staphylococcus saprophyticus and Enterococcus faecalis.
By GSK plc · Via Business Wire · March 25, 2025

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved PENMENVY (Meningococcal Groups A, B, C, W, and Y Vaccine) for use in individuals aged 10 through 25 years. The vaccine targets five major serogroups of Neisseria meningitidis (A, B, C, W, and Y) which commonly cause invasive meningococcal disease (IMD).1,2
By GSK plc · Via Business Wire · February 17, 2025
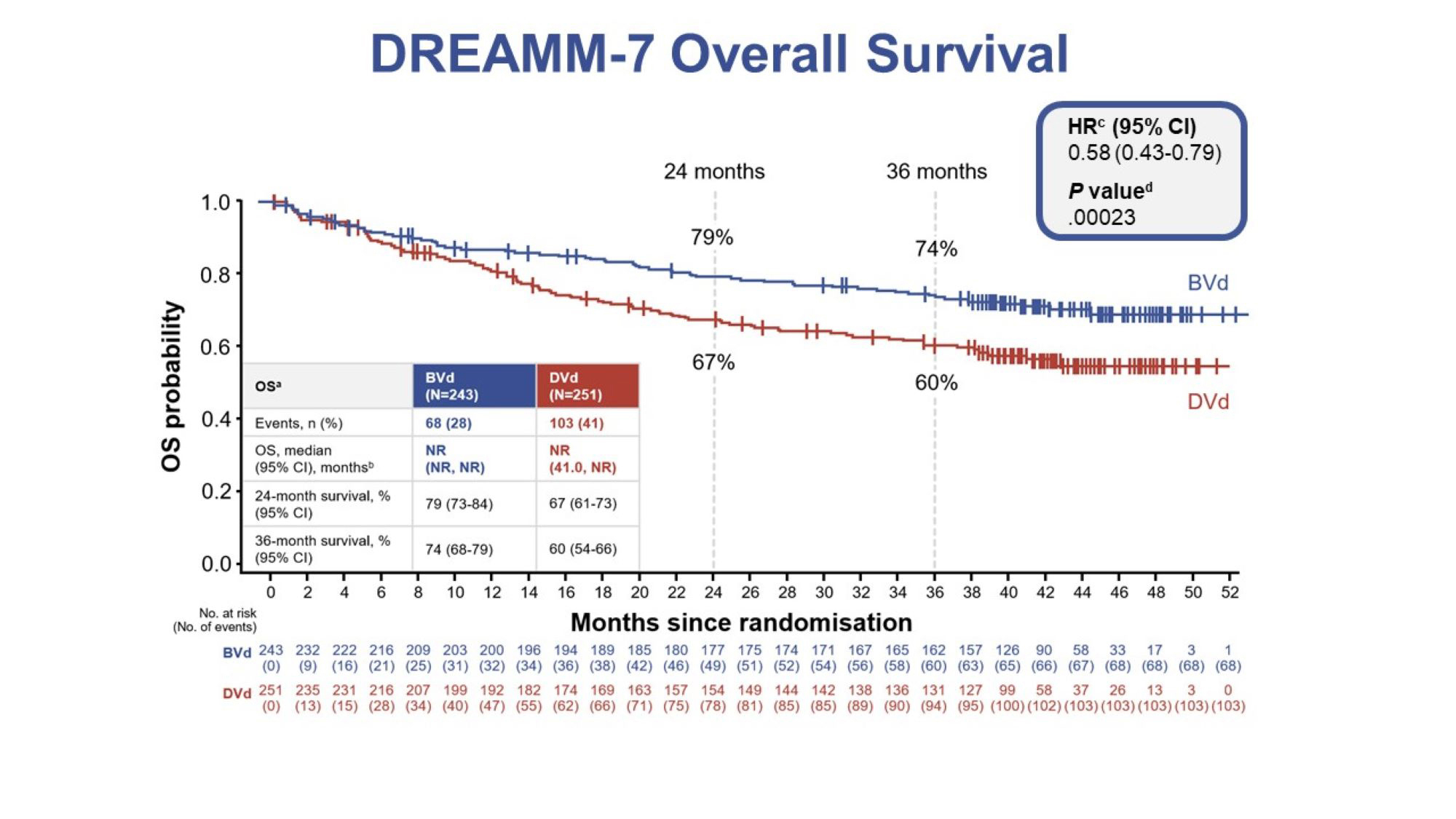
GSK plc (LSE/NYSE: GSK) today announced statistically significant and clinically meaningful overall survival (OS) results from a planned interim analysis of the DREAMM-7 trial evaluating belantamab mafodotin in combination with bortezomib plus dexamethasone (BVd) versus daratumumab in combination with bortezomib plus dexamethasone (DVd) as a second line or later treatment for relapsed or refractory multiple myeloma. These data were featured today in an oral presentation at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition.
By GSK plc · Via Business Wire · December 9, 2024

GSK plc (LSE/NYSE: GSK) today announced new preliminary data for AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) in adults aged 18-49 at increased risk for lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) due to certain underlying medical conditions and in adults who are immunocompromised. These data show the vaccine’s potential to help protect a broader group of adults at risk from the potentially serious consequences of RSV. In the US alone, the number of adults aged 18-49 with at least one risk factor that could put them at risk for RSV disease could exceed 21 million.1
By GSK plc · Via Business Wire · October 24, 2024

GSK plc (LSE/NYSE: GSK) today announced new data from the AReSVi-006 (Adult Respiratory Syncytial Virus) phase III trial evaluating the efficacy and safety of a single dose of AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) against lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in adults aged 60 years and older, including those at increased risk, over three full RSV seasons (NCT04886596).1 These data will be presented today at the CHEST 2024 Annual Meeting, organized by the American College of Chest Physicians.
By GSK plc · Via Business Wire · October 8, 2024

GSK plc (LSE/NYSE: GSK) today announced positive topline data from the phase 3 trial in adults 50 years and older evaluating the immunogenicity, reactogenicity and safety of AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) when co-administered with SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted), both AS01-adjuvanted vaccines (NCT05966090).1,2 The data were presented as a late-breaking abstract at the European Geriatric Medicine Society (EuGMS) Congress in Valencia, Spain (September 18-20th, 2024).2 SHINGRIX is approved for the prevention of shingles (herpes zoster) in adults aged 50 years and older. AREXVY is approved for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in individuals 60 years of age and older, as well as individuals 50 through 59 years of age who are at increased risk for LRTD caused by RSV.
By GSK plc · Via Business Wire · September 18, 2024

GSK plc (LSE/NYSE: GSK) today announced the US Food and Drug Administration (FDA) has approved Jemperli (dostarlimab-gxly) in combination with carboplatin and paclitaxel (chemotherapy) followed by Jemperli as a single agent for the treatment of adult patients with primary advanced or recurrent endometrial cancer. This approval broadens the previous indication for Jemperli plus chemotherapy to include patients with mismatch repair proficient (MMRp)/microsatellite stable (MSS) tumors who represent 70-75% of patients diagnosed with endometrial cancer and who have limited treatment options. The supplemental Biologics License Application (sBLA) supporting this expanded indication received Priority Review and was approved ahead of the Prescription Drug User Fee Act action date.
By GSK plc · Via Business Wire · August 1, 2024

GSK plc (LSE/NYSE: GSK) today announced it has started shipping doses of its trivalent influenza vaccines to US healthcare providers and pharmacies in preparation for the 2024-25 flu season. This immediately follows a licensing and lot-release approval from the US Food and Drug Administration (FDA).
By GSK plc · Via Business Wire · July 11, 2024

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved AREXVY (Respiratory Syncytial Virus (RSV) Vaccine, Adjuvanted) for the prevention of RSV lower respiratory tract disease (LRTD) in adults 50 through 59 years of age who are at increased risk. In the US, the vaccine is currently approved for use in adults aged 60 and older and recommended by CDC/ACIP using shared clinical decision-making.
By GSK plc · Via Business Wire · June 7, 2024

GSK plc (LSE/NYSE: GSK) today announced updated, longer-term results from the phase II supported collaborative study with Memorial Sloan Kettering Cancer Center (MSK) evaluating Jemperli (dostarlimab-gxly) as a first-line treatment—as an alternative to surgery—for mismatch repair deficient (dMMR) locally advanced rectal cancer. The trial showed an unprecedented 100% clinical complete response rate (cCR) in 42 patients who completed treatment with dostarlimab-gxly, defined as complete pathologic response or no evidence of tumors as assessed by magnetic resonance imaging, endoscopy and digital rectal exam. In the first 24 patients evaluated, a sustained clinical complete response with a median follow-up of 26.3 months (95% CI: 12.4-50.5) was observed.
By GSK plc · Via Business Wire · June 3, 2024

GSK plc (LSE/NYSE: GSK) today announced positive results from an interim analysis of the DREAMM-8 phase III head-to-head trial evaluating belantamab mafodotin, in combination with pomalidomide plus dexamethasone (PomDex), versus a standard of care, bortezomib plus PomDex, as a second line and later treatment for relapsed or refractory multiple myeloma. These late-breaking data, being presented today at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting (May 31 – June 4) in Chicago, IL, were featured in the official ASCO press program and simultaneously published in the New England Journal of Medicine.
By GSK plc · Via Business Wire · June 2, 2024

GSK plc (LSE/NYSE: GSK) today announced statistically significant and clinically meaningful overall survival (OS) results from Part 1 and progression-free survival (PFS) results from Part 2 of the RUBY/ENGOT-EN6/GOG3031/NSGO phase III trial in adult patients with primary advanced or recurrent endometrial cancer. These data were presented today in a late-breaking plenary session at the Society of Gynecologic Oncology 2024 Annual Meeting on Women’s Cancer (March 16-18).
By GSK plc · Via Business Wire · March 16, 2024

GSK plc (LSE/NYSE: GSK) today announced additional funding and new data and resources under the COiMMUNITY Initiative to help achieve higher adult vaccination rates and health equity in the US and address ongoing barriers to adult immunization. The COiMMUNITY Initiative complements recent industry and regulatory efforts to expand and improve adult vaccine availability, coverage and access.
By GSK plc · Via Business Wire · March 7, 2024

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has accepted under Priority Review an application to extend the indication of GSK’s adjuvanted respiratory syncytial virus (RSV) vaccine to adults aged 50-59 who are at increased risk for RSV disease. If approved, GSK’s RSV vaccine would be the first vaccine available to help protect this population. AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) is currently approved in the US in adults aged 60 and over for the prevention of lower respiratory tract disease (LRTD) caused by RSV.
By GSK plc · Via Business Wire · February 6, 2024

GSK plc (LSE/NYSE: GSK) today announced the recipients of the inaugural grant program of the COiMMUNITY Initiative, a multipronged effort to support the design of a more systematic, collaborative and equitable approach to helping increase adult immunization rates in the US. Each grant-funded project is receiving between $50,000 and $175,000 out of a total $1 million in funding to help address long-standing barriers to adult immunization in the US.
By GSK plc · Via Business Wire · December 12, 2023

GSK plc (LSE/NYSE: GSK) has partnered with former Queer Eye star and interior designer Thom Filicia to launch Mapping Myelofibrosis, a new health education initiative aiming to help those impacted by myelofibrosis (MF) better navigate the disease. This year marks the 10-year anniversary of Filicia donating bone marrow to his brother, who was diagnosed with MF a few months prior to the transplant. Filicia now looks to use his voice to help raise awareness of this blood cancer, which can be difficult to diagnose and manage.1
By GSK plc · Via Business Wire · November 13, 2023

GSK plc (LSE/NYSE: GSK) today announced positive preliminary results from its phase III trial [NCT05590403] evaluating the immune response and safety of AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) in adults aged 50 to 59, including those at increased risk of respiratory syncytial virus (RSV) lower respiratory tract disease (LRTD) due to certain underlying medical conditions. These results will be presented at the US Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) meeting on October 25, 2023. This vaccine is currently approved in the US for active immunization for the prevention of RSV-LRTD in adults 60 years of age and older. It is also approved in Europe, Japan, and several other countries.
By GSK plc · Via Business Wire · October 25, 2023

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved Ojjaara (momelotinib) for the treatment of intermediate or high-risk myelofibrosis, including primary myelofibrosis or secondary myelofibrosis (post-polycythemia vera and post-essential thrombocythemia), in adults with anemia. Ojjaara is a once-a-day, oral JAK1/JAK2 and activin A receptor type 1 (ACVR1) inhibitor. To date, it is the only approved medicine for both newly diagnosed and previously treated myelofibrosis patients with anemia that addresses the key manifestations of the disease, namely anemia, constitutional symptoms, and splenomegaly (enlarged spleen).4
By GSK plc · Via Business Wire · September 15, 2023

GSK plc (LSE/NYSE: GSK) and YMCA of the USA will host GSK’s Sideline RSV “Community Conversations” event series as part of their campaign efforts to raise awareness about RSV infection in older adults. The events will take place at four YMCA locations across the country (Chicago, Los Angeles, New York City and Phoenix), joined by GSK’s campaign spokesperson Earvin “Magic” Johnson, to help spark important conversations about RSV, a common, contagious respiratory virus.1,2 Kicking off in September and running through RSV Awareness Month in October, the private events will include perspectives from medical professionals, and patients and convene public health leaders, media, and 60+ YMCA members.
By GSK plc · Via Business Wire · September 5, 2023
GSK plc (LSE/NYSE: GSK) today announced that AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) is now available in the US at all major retail pharmacies. In June, the Advisory Committee on Immunization Practices (ACIP) recommended that persons 60 years of age and older may receive a single dose of RSV vaccine, using shared clinical decision making. Shared clinical decision making empowers patients, in consultation with their healthcare providers, to decide whether RSV vaccination is appropriate for them. AREXVY is indicated for the prevention of RSV-lower respiratory tract disease (LRTD) in individuals aged 60 years and older.
By GSK plc · Via Business Wire · August 17, 2023

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved Jemperli (dostarlimab-gxly) in combination with carboplatin and paclitaxel, followed by Jemperli as a single agent for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR), as determined by an FDA-approved test, or microsatellite instability-high (MSI-H). The supplemental Biologics License Application (sBLA) supporting this new indication received Priority Review and was approved ahead of the Prescription Drug User Fee Act action date.
By GSK plc · Via Business Wire · July 31, 2023

GSK plc (LSE/NYSE: GSK) today announced it has started shipping doses of its quadrivalent influenza vaccines to US healthcare providers and pharmacies in preparation for the 2023-24 flu season. This immediately follows a licensing and lot-release approval from the US Food and Drug Administration (FDA).
By GSK plc · Via Business Wire · July 13, 2023

GSK plc (LSE/NYSE: GSK) today announced that the US Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted in favor of recommending the use of AREXVY (respiratory syncytial virus vaccine, adjuvanted) in adults aged 60 and older using shared clinical decision making. Shared clinical decision making empowers patients in consultation with their healthcare providers to determine whether RSV vaccination is appropriate for them.
By GSK plc · Via Business Wire · June 21, 2023

GSK plc (LSE/NYSE: GSK) today announced new data from the AReSVi-006 (Adult Respiratory Syncytial Virus) phase III trial evaluating the efficacy of a single dose of AREXVY (respiratory syncytial virus vaccine, adjuvanted) against lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in adults aged 60 years and older over multiple RSV seasons and after annual revaccination.
By GSK plc · Via Business Wire · June 21, 2023

GSK plc (LSE/NYSE: GSK) announced today the launch of a new short film in collaboration with Lifetime that raises awareness among parents of teens and young adults about meningitis B vaccination, and the potential risks of meningitis B. The film, entitled “I Never Thought to Ask: A Mom's Quest for Answers” with Soleil Moon Frye Brought to You By GSK” takes viewers on a journey of discovery, as Soleil connects with a medical professional, and individuals and families affected by the disease – learning about important questions parents can ask their teen’s doctor.
By GSK plc · Via Business Wire · June 20, 2023

GSK plc (LSE/NYSE: GSK) today announced the launch of the COiMMUNITY Initiative to help reduce health inequities and set a new precedent for adult immunization rates in the US, which continue to remain below pre-pandemic levelsi. The initiative is a multipronged effort to address long-standing barriers to adult immunization in the US with funding, increased data transparency through enhanced Vaccine Track capabilities and collaborations, and resource-sharing opportunities.
By GSK plc · Via Business Wire · June 1, 2023
GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved AREXVY (respiratory syncytial virus vaccine, adjuvanted) for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in individuals 60 years of age and older. This is the first RSV vaccine for older adults to be approved anywhere in the world.
By GSK plc · Via Business Wire · May 3, 2023

GSK plc (LSE/NYSE: GSK) has partnered with Earvin “Magic” Johnson to launch Sideline RSV, a new health education campaign aimed to help older adults and their loved ones better understand the risks and potential seriousness of RSV infection and how to help protect themselves.
By GSK plc · Via Business Wire · March 14, 2023

GSK plc (LSE/NYSE:GSK) today announced new 48-week data from the MOMENTUM phase III trial that showed a majority of patients treated with investigational momelotinib maintained their responses across key clinical measures including Total Symptom Score (TSS), Transfusion Independence (TI) rate, and Splenic Response Rate (SRR) in myelofibrosis patients previously treated with an approved Janus kinase (JAK) inhibitor. Additionally, new analyses from MOMENTUM showed that TI response with momelotinib at week 24 was associated with overall survival. These data were presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition (10-13 December) in New Orleans.
By GSK plc · Via Business Wire · December 11, 2022

GSK plc (LSE/NYSE: GSK) today announced the launch of Vaccine Track, a comprehensive platform developed by GSK and IQVIA for use by public health officials, industry leaders and medical professionals to strengthen vaccination data transparency, raise awareness and publicly share vaccination trends to aid improvements in routine adult vaccinations to create healthier communities across the US. This resource will provide frequent and relevant data on trends to focus and enhance public health efforts.
By GSK plc · Via Business Wire · August 8, 2022

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved Benlysta (belimumab) for the treatment of children aged 5 to 17 with active lupus nephritis (LN) who are receiving standard therapy. Lupus nephritis is a serious inflammation of the kidneys caused by lupus, which can lead to end-stage kidney disease, requiring dialysis or a kidney transplant.i The approval extends the current indication in the US to include both lupus and active LN for the intravenous formulation in the pediatric patient population.
By GSK plc · Via Business Wire · July 27, 2022

GSK plc (LSE/NYSE: GSK) today announced it has started shipping doses of its quadrivalent influenza vaccines to US healthcare providers and pharmacies in preparation for the 2022-23 season. This immediately follows a licensing and lot-release approval from the US Food and Drug Administration’s (FDA) Center for Biologics Evaluation and Research.
By GSK plc · Via Business Wire · July 11, 2022

GSK plc today announced the first recipient of the Target the Future Think Tank Challenge £70,000 (equivalent to approximately $100,000) grant to the HealthTree Foundation, a non-profit organization helping patients learn more about their health and become their own best advocates. Their proposal, the “HealthTree Equity and Diversity for Multiple Myeloma Program,” will improve access, education and support for underserved communities and minority patients.
By GSK plc · Via Business Wire · June 29, 2022

GSK plc (LSE/NYSE: GSK) today announced that the US Food and Drug Administration (FDA) has approved PRIORIX (Measles, Mumps and Rubella Vaccine, Live) for active immunisation for the prevention of measles, mumps and rubella (MMR) in individuals 12 months of age and older.
By GSK plc · Via Business Wire · June 6, 2022
